phosphorus electron affinity|7.5: Electron Affinities : Pilipinas Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative . Salt: The continuing adventures of CIA agent Evelyn Salt.
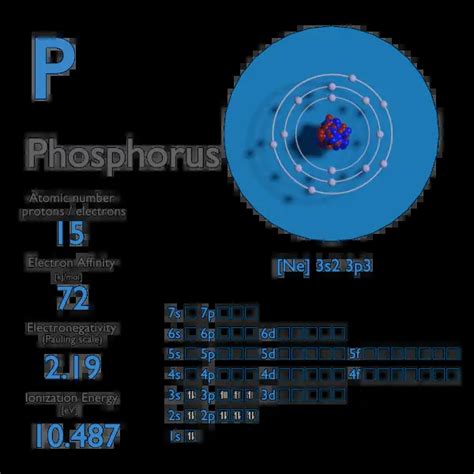
phosphorus electron affinity,Electron affinity of Phosphorus is 72 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule .
Ago 11, 2023 Electron Affinity of Phosphorus is 72 kJ/mol. Electronegativity of Phosphorus is 2.19. First Ionization Energy of . Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative .
The electron affinity is defined as the amount of energy released when an .phosphorus electron affinityAtomic spectrum. A representation of the atomic spectrum of phosphorus. Ionisation Energies and electron affinity. The electron affinity of phosphorus is 72 kJ mol ‑1. The ionisation energies of phosphorus .The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy .Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to ., The electron affinity of phosphorus, J. Phys. B - Atom. Mol. Opt. Phys., 2007, 40, 20, 4097-4107, https://doi.org/10.1088/0953-4075/40/20/010. Slater and Linberger, 1977 . Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) . We have measured the energies of all three fine structure components in the 3 P J ground state of the negative ion of phosphorus using laser photodetachment threshold spectroscopy. The experiment yielded an electron affinity of 746.68(6) meV. The ΔJ = 2–0, 2–1 and 1–0 fine structure splittings were determined to be 32.73(7) meV, 22.48(7) meV .phosphorus electron affinity 7.5: Electron Affinities The experiment yielded an electron affinity of 746.68(6) meV. The J 2–0, 2–1 and 1–0 fine structure splittings were determined to be 32.73(7) meV, 22.48(7) meV and 10.25(3) meV, respectively. In the experiment, a mass selected beam of P− ions was merged with the output from a pulsed infrared optical parametric oscillator.Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy . This electron pairing requires additional energy and thus it is easier to add electrons if there are free orbitals. When element has a half-filled p sublevel all 3 orbitals have one electron and pairing takes place (difference between energy levels of 2p and 3s is greater than electron pairing energy).
The electron affinity [EA] is the energy change for the process of adding an electron to a gaseous atom to form an anion (negative ion). X(g) +e− X−(g) EA1 (3.4.1) (3.4.1) X ( g) + e − X − ( g) EA 1. This process can be either endothermic or exothermic, depending on the element. The EA of some of the elements is given in Figure 3.4.6 3.4.

Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. . Phosphorus: 0.746 609(11) 72.037(1) 16 32 S . The adiabatic electron affinities of five second row atoms (Al, Si, P, S, Cl) and their monoxides and dioxides were determined using six different density . Electron affinities of the oxides of aluminum, silicon, phosphorus, sulfur, and chlorine Nicole R. Brinkmann; Nicole R. Brinkmann Center for Computational Quantum Chemistry, . Phosphorous atoms have weaker electronegativity, with an electron affinity ( EA phos ) = 0.746 eV [19, 20] compared to oxygen atoms ( EA oxygen = 1.47 eV ) [21]. The work function of black .
The electron affinity of group 4 elements is more than that of group 5 elements. This is because of the p orbitals of these elements. For example, phosphorous has its 3P orbital half-filled which is fairly stable so when one more electron is accepted the stability is disturbed. This is why the electron affinity of phosphorous is less than silicon.7.5: Electron Affinities Other names: red phosphorus; Phosphorus atom Permanent link for this species. Use this link for bookmarking this species for future reference. . Electron affinity determinations. EA (eV) Method Reference Comment; 0.746609 ± 0.000009: N/A: Pelaez, Blondel, et al., 2011: given: 0.716607(10) eV; B:
The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on .Definition: Electron Affinity of an Atom. The electron affinity of an atom is the energy released when an electron is added to a neutral atom in the gas state to form a negative ion, per mole of atoms. Electron affinities are usually expressed in kilojoules per mole ( kJ/mol ). We also use the word electron affinity to refer to the overall . Black phosphorus is likely to be the layered semiconductor material with the highest carrier mobility at room temperature, making it promising for high-performance electronic applications.
phosphorus. Formula: P. Molecular weight: 30.973762. IUPAC Standard InChI:InChI=1S/P Copy. IUPAC Standard InChIKey:OAICVXFJPJFONN-UHFFFAOYSA-N Copy. CAS Registry Number: 7723-14-0. Chemical structure: This structure is also available as a 2d Mol file. Other names: red phosphorus; Phosphorus atom. Permanent link for this species.
Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form F⁻(g), the associated energy change is -328 kJ/mol.Because this value is negative (energy is released), we say that the electron affinity of fluorine is favorable. Created by Jay.

In chemistry, the electron affinity of an atom of Phosphorus is defined as the amount of energy released or spent when an electron is added to a neutral atom in the gaseous state to form a negative ion. But what is the electron affinity of an atom of P? In the case of Phosphorus the electron affinity is 72 kilojoules per mole.The electronic affinity is amount of energy, that is released during the attachment of the electron to the neutral atom. As a result of such attachment, a negative ion (anion) is formed. Electron affinity is related to electronegativity of elements.Simply speaking, the greater the affinity of electrons, the more eagerly the atoms of a given element join .Compare Phosphorus vs Silicon of the Periodic Table on all their Facts, Electronic Configuration, Chemical, Physical, Atomic properties. . Ionization Energies and electron affinity. Name: Phosphorus: Silicon: Valence or Valency: 5 : 4 : Electronegativity: 2.19 Pauling Scale: 1.9 Pauling Scale: Electron Affinity: 72 kJ/mol: 133.6 kJ/mol:
phosphorus electron affinity|7.5: Electron Affinities
PH0 · phosphorus
PH1 · Phosphorus
PH2 · Electron affinity (data page)
PH3 · Electron Affinity Chart (Labeled Periodic table + List)
PH4 · Electron Affinity
PH5 · A7: Electron Affinities
PH6 · 7.5: Electron Affinities